Regulatory Requirements
The Institutional Official
Institutional Animal Care and Use Committee (IACUC)
The Attending Veterinarian
Regulatory Requirements
Research with animals is guided by a complex set of federal and state laws, regulations, and guidelines, and by additional institutional policies implemented to systematically address federal requirements and as the result of a voluntary accreditation process. In addition to these laws and regulations, researchers and animal care technicians are guided by ethical considerations for the proper care and use of animals. Which laws and regulations apply depends, in part, on the species of animal and source of the research funding.
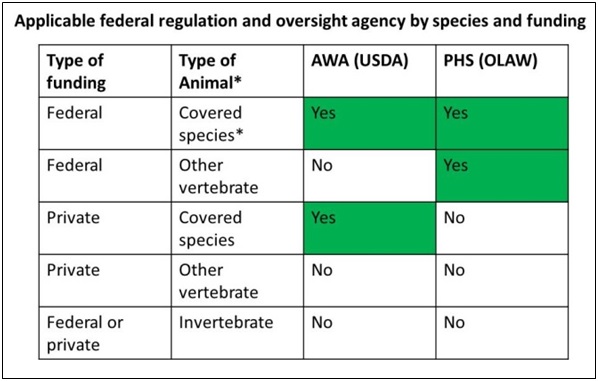
The Public Health Service Policy on Humane Care and Use of Animals[1](PHS Policy) and USDA Animal Welfare Regulations[2] (AWRs) provide the primary regulatory basis for the existence and function of the Institutional Animal Care and Use Committee (IACUC), which must be established at any institution that receives PHS funding for vertebrate animal-based research, and/or which conducts animal-based research involving vertebrate animal species regulated by the USDA AWRs[3].
PHS Policy requires that all institutions base their animal care and use programs on the Guide for the Care and Use of Laboratory Animals[4] (the Guide), and that euthanasia be consistent with the most current American Veterinary Medical Association (AVMA) Guidelines on Euthanasia[5]. The PHS Policy also endorses the US Government Principles for the Utilization and Care of Vertebrate Animals Use in Testing, Research and Training[6], which form the foundation for ethical and humane care and use of laboratory animals in the United States.
Many institutions are also subject to additional standards that go above and beyond the regulatory requirements by maintaining accreditation through the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International. Though accreditation is voluntary, many institutions choose to become AAALAC accredited as a way of demonstrating their high standards of animal care
Depending on the nature of a protocol (for example, studies involving biohazardous agents, radioactive materials, or threatened/endangered animal species), other federal, state, and local laws, regulations, and policies may also be applicable.
The regulations are set forth with the ultimate goal of ensuring replicable, generalizable research data. This, in turn, requires standardization of care and humane treatment of animals. Stressed or unhealthy animals make poor study subjects–they do not provide good data and can introduce uncontrolled variability in the research results. If valid, reproducible data cannot be obtained, the use of animals cannot be ethically justified. The established laws and regulations, therefore, function to prescribe a commonly understood basis for ensuring that humane standards are maintained for the care of animals.

Each institution must identify an Institutional Official (IO) who is legally authorized to commit, on behalf of the institution, that the requirements of the PHS Policy and USDA AWRs will be met. The IO is appointed by the Chief Executive Officer of each institution and must be assigned institutional responsibility to commit the financial and other resources to ensure compliance with the governing regulations, as well as to initiate and/or codify institutional policies and procedures to promote high-quality science and animal well-being. The IO does not need to be a scientist or
work with animals, but must be someone who will accept responsibility for the wellbeing of the animals.”
The IO is responsible for providing general oversight and direction of the institutional animal care and use program. This includes taking the lead in creating a dynamic, compliant, and responsible institutional culture, and establishing an expectation of quality scientific research while promoting the humane use and well-being of animals used in research. To accomplish these goals, the IO must appoint and empower key personnel to provide day-to-day oversight of the program as well as the resources required to ensure quality science and promote animal well-being. The IO is additionally designated the responsibility to appoint members to the IACUC.
The Institutional Animal Care and Use Committee (IACUC)
The IACUC reports to the Institutional Official, as required by federal regulations. The Committee’s specific responsibilities are spelled out in PHS Policy IV.B and USDA AWRs section 2.31(c):
1. The IACUC must review the institution’s program for humane care and use of animals at least once every six months, using the Guide and USDA AWRs as the basis for evaluation.
2. The IACUC must inspect all of the institution’s animal facilities at least once every six months, using the Guide and USDA AWRs as the basis for evaluation. “Animal facilities” includes any and all buildings, rooms, areas, enclosures, or vehicles, including study areas[7], used for animal confinement, transport, maintenance, breeding, or experiments inclusive of surgical manipulation.
The IACUC is also responsible for preparing reports of the evaluations as described above, and submitting the reports to the Institutional Official. The reports must contain a description of the nature and extent of the institution’s adherence to the Guide, PHS Policy, and USDA AWRs, and must specifically identify any departures from these regulations. If program- or facility-related deficiencies are noted, the reports must contain a reasonable and specific plan and schedule for correcting each deficiency.
3. The IACUC is responsible for reviewing and, if warranted, investigating concerns involving the care and use of animals at the institution. These concerns may be identified by members of the IACUC during routine program reviews and inspections, or may be the result of public complaints or reports of noncompliance from laboratory or research facility personnel.
4. The IACUC is authorized to make recommendations to the Institutional Official regarding any aspect of the institution’s animal program, facilities or personnel, to ensure regulatory compliance.
5. The IACUC must review and approve, require modifications in (to secure approval), or withhold approval of proposed activities related to the care and use of animals. Once approved, all activities are subject to ongoing review at least annually, with a complete re-review required every three years. The following general criteria must be considered during the review of animal use protocols:
- Rationale and purpose of the proposed use of animals.
- Justification of the species and number of animals requested. Whenever possible, the number of animals requested should be justified scientifically.
- Availability or appropriateness of the use of alternatives to the use of animals, including less-invasive procedures, other species, isolated organ preparation, cell or tissue culture, or computer simulation.
- Adequacy of training and experience of personnel in the procedures used.
- Unusual housing and husbandry requirements.
- Appropriate sedation, analgesia, and anesthesia.
- Unnecessary duplication of experiments.
- Conduct of multiple major operative procedures.
- Criteria and process for timely intervention, removal of animals from a study, or euthanasia if painful or distressful outcomes are anticipated.
- Post-procedural care, including methods to protect animal well-being and to minimize post-operative pain/distress.
- Method of euthanasia of disposition of animal.
- Safety of working environment for personnel.
6. Similarly, the IACUC is required to review and approve, require modifications in (to secure approval), or withhold approval of proposed significant changes regarding the use of animals in ongoing activities. The IACUC uses the same criteria described above to assess proposed significant changes.
7. Finally, the IACUC must be authorized to suspend an activity involving animals if the IACUC determines the activity is not being conducted in accordance with the approved research protocol or with other applicable provisions of the USDA AWRs, the Guide, the institution’s Assurance, or PHS Policy. In the event an activity is suspended, the IACUC and the Institutional Official will review the reasons for the suspension and determine the appropriate corrective actions.
The Attending Veterinarian
Adequate veterinary medical care is an essential component of any research program and is mandated by both the PHS Policy and USDA AWRs. The Attending Veterinarian (AV) is the individual with legal responsibility for the health and welfare of animals at the research facility. To fulfill this legal accountability, the veterinarian has the authority to make decisions on behalf of the animals in the event that their health or welfare becomes compromised. The Campus Veterinarian assumes the role of the AV but may delegate many of his responsibilities to other veterinary staff.
An adequate veterinary care program must include a number of components, including: (1) sufficient facilities, equipment, personnel, and infrastructure to provide adequate care; (2) daily observation of all animals to assess their health and well-being; (3) availability of emergency, weekend, and holiday animal care; (4) adequate guidance and training of personnel who care for animals; (5) provisions for appropriate pre-and post-procedural animal care; (6) use of appropriate methods to prevent, control, diagnose, and treat diseases and injuries; and (7) providing training in techniques for animal handling, aseptic surgical technique, and other standard research procedures.
[1] http://grants.nih.gov/grants/olaw/references/PHSPolicyLabAnimals.pdf
[2] https://www.aphis.usda.gov/animal_welfare/downloads/Animal%20Care%20Blue%20Book%20-%202013%20-%20FINAL.pdf
[3] USDA regulations apply to all mammals, with the exception of laboratory mice and rats bred for the purpose of research.
[4] http://www.nap.edu/readingroom/books/labrats/
[5] http://www.avma.org/issues/animal_welfare/euthanasia.pdf
[6] http://grants.nih.gov/grants/olaw/references/phspol.htm#USGovPrinciples
[7] A study area is defined as any investigator-managed building, room, area, enclosure, or other containment site in which USDA-regulated animals are housed for periods longer than 12 hours, or in which non-USDA-regulated animals are housed for periods longer than 24 hours.
