 Teresa Romeo Luperchio is a graduate student at The Johns Hopkins University School of Medicine and is currently working as a FASEB Office of Public Affairs Fellow. In this post Teresa tackles the difficult question of why cell lines could not replace all animal studies.
Teresa Romeo Luperchio is a graduate student at The Johns Hopkins University School of Medicine and is currently working as a FASEB Office of Public Affairs Fellow. In this post Teresa tackles the difficult question of why cell lines could not replace all animal studies.
A common theme in alternatives to animal testing is that tissue culture, or growing cells in a dish, is a viable way to replace animals in research. And for sure, growing cells in dishes for experiments is a great system; cells can be easily grown, the number of cells can be expanded to accommodate the size of your experiment, and they grow in small containers you can work with at the bench. Researchers can also buy cell lines (derived from animals or humans) from a company or can borrow cells from a colleague and grow them up in their own laboratory. Advances in culture techniques have made using cell lines an easy way to study almost every topic that relates to health and disease. However, often overlooked or forgotten is the real identity of these cells.
Tissue culture in the lab
Cells, when still in the body, are limited in the number of times they can divide to make new, daughter cells. This phenomenon, known has Hayflick’s limit, is critical. Bypassing this limit can lead to uncontrolled growth and cancer. In culture, however, it is typical to develop cell lines that grow indefinitely, occurring through a process called immortalization. When normal cells are prepared for culture, the process is the same regardless of tissue type: 1) cells and tissues are extracted from an animal, 2) the tissue is ground up (homogenized) as necessary to produce single cells, and 3) the cells are provided the nutrients and chemicals they need to grow and divide in culture dishes. To “immortalize” the cells, scientists use a number of different methods to trick them into growing past their natural Hayflick limit, which normal cells in culture rarely meet. The end result is an immortalized cell line that provides researchers with an almost endless amount of material.
Immortalized cell lines, once established, are a powerful tool for experimental studies, but they are a distinct cell type with distinct features. Not only do the populations of cells continue to expand, but their metabolism, the way the cells communicate with each other, and even their genomes can change. At our peril, the research community forgets, and groups promoting tissue culture research as an alternative for using animals ignore this caveat. Eliminating tissue culture, however, is an unfair assessment. In fact, much great science has been performed using immortalized cell lines, and in some cases, their use is preferred when variability and comparison to normal tissues are not a concern.
One way to avoid some of the pitfalls of using immortalized cell can be to use primary cell cultures, or cells immediately grown from a donor organism. These cells are self-limiting in growth number and are generally short term cultures. They do reduce the numbers of animals needed in research because scientists are able to freeze cells for future use like immortalized lines, but every new experiment that needs a new line would require another cell or tissue donor. Elimination of animals in research would hinder the ability to study relevant biology for normal and disease states even in tissue culture models.
Acknowledge the problems
As research tools become more powerful and more exact, small variations in our experiments are becoming more obvious when analyzing data. This increased sensitivity and scrutinizing power is fantastic for scientific exploration but also sobering in that we are becoming more aware and more cautious of our experimental materials. The media has picked up issues of ‘reproducibility’ in science, especially relating to cell lines. As many as 36% of cell lines are not what they are labeled to be or are contaminated with other cell types and this can lead to confounding results. Sharing cell lines can lead to confusion and mislabeling of cell types and sub-culturing cells can introduce variability. The National Institutes of Health have also picked up on this issue plaguing science research and is working on providing support and guidelines for scientists using cell lines to minimize error or misinterpretation. Science is self-correcting, and each experiment imparts new information that shapes global health and wellness. Keeping animals in research and employing the use of primary cultures eliminates some of the inconsistencies and variability between experiments using immortalized lines.
Examples of the need for primary cells
Genomic studies are the new boom of science and medicine. Improved, faster and cheaper imaging, computing, and sequencing technologies have accelerated genomic research. Genomic studies have led to many breakthroughs in disease and revolutionized biotechnology and academic research.
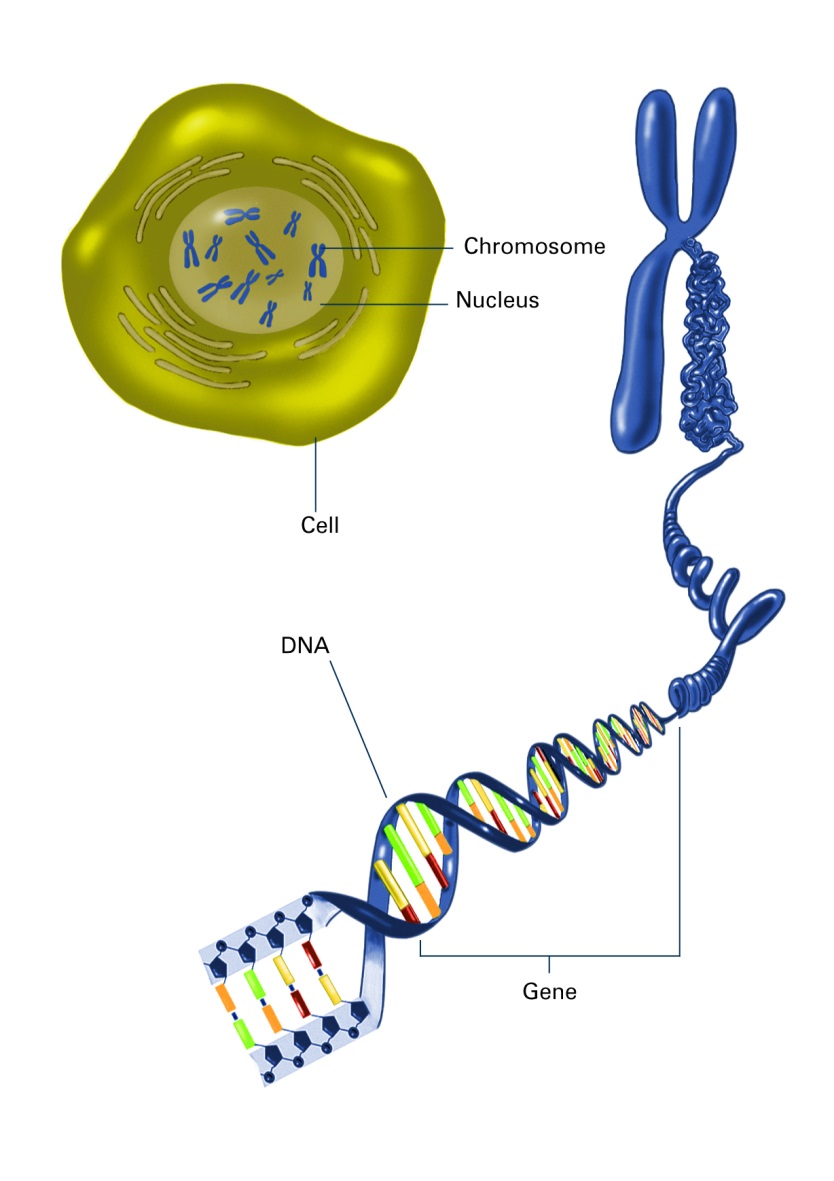
Generally, the genome is thought of as linear, with genes located next to each other in a long string connected end to end. When there are changes to DNA, we imagine mutations to the sequence or breaks in that long ribbon (This year, the Nobel Prize in Chemistry was awarded to three researchers who discovered repair mechanisms for the genome). DNA is packaged into chromosomes, and all that genetic material is bundled into the cell’s nucleus. It is becoming clear that how it is folded and packaged in the nucleus can influence disease. The organization of your genetic material is complex, decided by a vast array of proteins and structures within the nucleus, and it is constantly changing to allow for genes to be expressed or not expressed. While DNA sequence generally remains unchanged, gene position, organization of chromosomes and as well as epigenetic signatures are affected by changes to the environment, and these impact gene expression and can trigger disease.
Tissue culture can impact the genome and create confusion in results. When cell lines are generated, the end product does not resemble an organism at all. The genome becomes unstable and prone to mistakes, and after a few cycles of growth, or passes, the cell lines may not even resemble what you started with. We often see in culture that cells become polyploid (have more chromosomes than they should), which is characteristic of cancer.
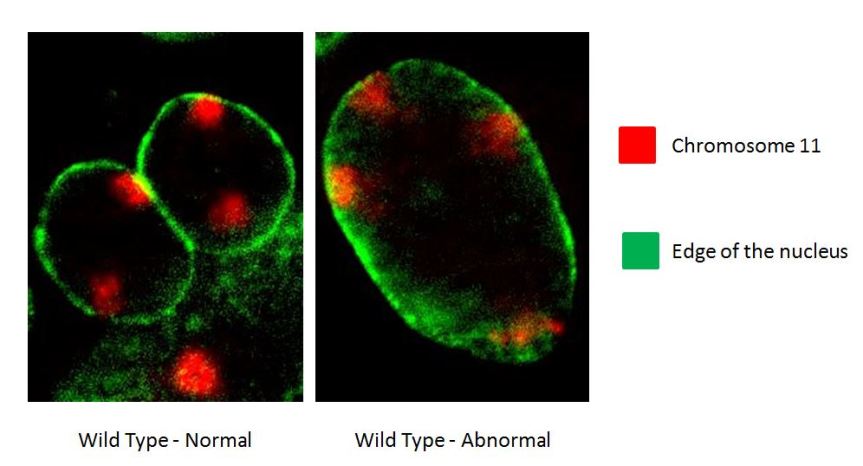
For this reason, to understand how the genome and epigenome behave in normal conditions and to study diseases that afflict humans and animals, using established immortalized cell lines can lead to confounding or irreproducible results. When using primary cells and limiting the time we study them in culture, we can eliminate the issue of unstable cell lines. In this case, the cells more closely resemble the cells in the body, genetically and in behavior, and they provide more realistic and applicable data. Primary cells are not immune to increases of ploidy or culture induced instability and metabolic changes. After time in culture, often-times even after only weeks, they also show signs of diverging from their original characteristics, similar to immortalized cell lines. Limiting the time in culture reduces the effects of tissue culture on the identity of primary cells, but requires a researcher to return to the tissue source once the population shows characteristics of population drift and for each new line or experiment. While primary cells that replace immortalized lines are more tedious to rely on because you must continue to return to an animal, they still can provide the opportunity for massive expansion of cells, which reduces the number of animals in research and allows for researchers to do basic science studies quickly, efficiently and effectively without compromising validity.
Remember where cells come from
Regardless of immortalized or not, cells used in tissue culture come from a donor. In the case of humans, they are often procured during biopsies or are excess material during surgeries. The amount of material from humans is small and limited. The use of animals expands the capacity to test and fully understand treatments that impact human and animal health in a way that would be impossible using products from human biopsy. While many primary cultures can be grown for a few weeks and some can be immortalized, there are many cell types such as neural tissues that are terminally differentiated (i.e., cannot replicate themselves in a dish), and must be taken from a donor to do experiments outside the body. This is an important point to note as there are no models of cell culture, immortalized or not, that will be able to eliminate animals when studying these body systems.
Other cell systems, such as induced pluripotent stem cells (iPSCs), have been suggested as an alternative source to using animals in research because they can potentially produce all cell types.iPSCs can be created from cells non-invasively obtained from a human or animal model, and then coerced to grow into cell types that are typically harvested from animals for studies. While iPSCs can potentially reduce the numbers of animals in research, iPSC populations are also plagued with issues of identity. It has been reported that they often retain some signatures and features of their originating tissue types. Cells and tissues directly from a donor is the only way to be sure of cellular identity and ensure the most robust and relevant results.
Conclusion
Tissue culture is a powerful system that has led to significant scientific advances that have positively impacted human health and reduces the number of animals in research. The goals of using basic models such as cells in research is to create and discover strong, relevant and reproducible data that can eventually be used to diagnose and treat diseases that impact both humans and animals. By ensuring the quality of our reagents in doing even the most basic research, we ensure quality data that benefits the scientific and general community at large. Removing animals in research limits the ability for researchers to discover new and effective therapies that impact human health.
Teresa Romeo Luperchio
