In neurological research the importance of neuronal death is well known, as are its implications for the normal functions of your brain. Many serious neurological conditions, such as stroke, epilepsy, traumatic brain injuries, and degenerative diseases like Parkinson’s and Huntington’s Chorea, are a direct consequence of this type of neuronal death, which determines the clinical manifestation of the illness and, as a consequence, the prognosis for the patient.
For example, in stroke an ischemic attack (loss of blood flow), even if it is localized, can initiate the neuronal death program, due to excessive stimulation of neurons (nerve cells) by molecules known as neutotransmitters, the so-called excitotoxic triggers. This in turn leads to a worse clinical development and physical symptoms like palsies, sensory alterations, cognitive impairments and more.
One of the principal triggers of this event is the activation of the N-methyl-D-aspartate receptors on neurons, which are sensitive to glutamic acid, one of the most important excitatory neurotransmitter of the brain.
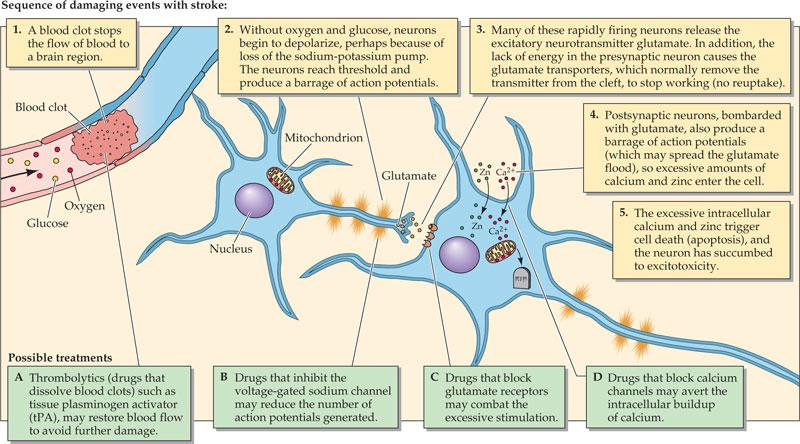
When these receptors are over activated due to the release of an enormous amount of glutamic acid as a consequence of the lack of oxygen in the affected area of the brain, this triggers a flow of Ca++ ions into the neuron, which activates many enzymes inside the nerve cell that directly damage the internal structure and ultimately cause the cells to die in a process known as apoptosis. (1)
It’s clear at this point how important it is to stop this process in order to prevent progressive damage in areas that are not directly affected by the original ischemic event.
What’s still not so clear is the precise molecular pathway from the liberation of glutamic acid to the neuronal death, but experimental evidence suggests that a membrane protein called JNK (c-Jun-N-terminal-kinase) has an important role in this activation. (2)
Although it has been shown that by blocking JNK it’s possible to reduce infarct size (the size of the damaged area of brain tissue) and neuronal death in an in vivo animal model of cerebral ischemia, the same studies have also shown serious side effects, as JNK has several important physiological functions in the body. As a result researchers have sought to identify means of blocking the role of JNK in ischemia–induced neuronal cell death without blocking its other functions.
It has been demonstrated that JNK is activated by two upstream molecules, called respectively MKK4 and MKK7, that respond to specific stress situations and represent a key bottleneck, and that in particular MKK7 shows an important role as mediator in the activation of JNK as ain response to cerebral ischemia.
Due to this observation, a team of neuroscience Italian researchers developed a specific MKK7 inhibitor peptide, called GADD45ß-I, to study its possible effects in vitro and in vivo in rodent models.(3)
This molecule showed an interesting neuroprotective effect in vitro and no toxicity itself on neurons, suggesting its application for in vivo treatments; during the in vivo (animal research) phase, the molecule was tested on two different rodent models which demonstrated that this peptide could reduce the infarct area of 43% after 24h, if administrated before the induced stroke. Very importantly this neuroprotective effect was still maintained when GADD45ß-I was administered 6h after the initial ischemic damage, which is critical as analysis of earlier failed candidate stroke therapies have stressed that potential therapies must be able to prevent damage when administered several hours after stroke onset (when treating stroke prompt diagnosis and treatment is vital). These protective effects are maintained for at least a week and show that the molecule does not merely delay apoptosis but actively blocks the process.
To prevent ischemic damage in the immediate aftermath of stroke onset, we can use rt-PA (recombinant tissue plasminogen activator) to promote the breakdown of a possible obstruction inside a cerebral artery and prevent a progressive stroke; and this is an important approach that has saved many lives in the last 20 years. However, this therapy has side effects such as bleeding, and it can be not use in some specific but common conditions, for example in patients who use anticoagulant as Warfarin for atrial fibrillation, and is only effective if administered within 3,5-4,5 hours of stroke onset (although it may be effective later in some cases where the damaged area is clearly demarcated in brain imaging by MRI or CT scan).
All other therapies that are available to neurologists are only supporting therapy for the blood pressure, active anticoagulation and respiratory support where it is necessary.
GADD45ß-I offers the possibility that we could protect a patient under ischemic insult even when we could not use the thrombolytic therapy with rt-PA, and we could also protect them from future insults by regular administration of this drug, which may be especially useful for multimorbidity patient, those who suffer from two or more chronic health conditions.
This could also lead to reduce post-stroke consequences, to improve the prognosis for these persons and to a reduced need for rehabilitative therapies as physiotherapy, speech therapy and exercise therapy
If these promising early results are confirmed in clinical trials, this therapy could be one of the most important discoveries in the field of neurology in the recent years and could radically change our approach on stroke, allowing us to switch from a supportive therapy to a preventative therapy.
If we think that in 2010 circa 17 million stroke occurred worldwide, and that every 6 seconds a person somewhere suffers a stroke, we can also imagine the potential impact of this therapy.
- Lipton SA. Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat Rev Drug Discov 2006; 5: 160–170.
- Centeno C, Repici M, Chatton JY, Riederer BM, Bonny C, Nicod P et al. Role of the JNK pathway in NMDA-mediated excitotoxicity of cortical neurons. Cell Death Differ 2007; 14: 240–253.
- Vercelli A, Biggi S, Sclip A, Repetto IE, Cimini S, Falleroni F, Tomasi S, Monti R, Tonna N, Morelli F, Grande V, Stravalaci M, Biasini E, Marin O, Bianco F, di Marino D, Borsello T. Exploring the role of MKK7 in excitotoxicity and cerebral ischemia: a novel pharmacological strategy against brain injury. Cell Death Dis. 2015 Aug 13;6: e1854.
Marco
